The mechanics of chlorine disinfection are, of course, covered in PWTAG’s book Swimming Pool Water, and in its Code of practice. This technical note goes somewhat beyond the two in its detail. It should reinforce those two sources, as well as giving fresh insights, including into the mechanics of microbiological action.
The basic chemistry
When sodium or calcium hypochlorite are added to water, hypochlorous acid (HOCl) also known as active free chlorine and hypochlorite ion (OCI-) are formed. Together these are known as free chlorine. Of the two, HOCl is the form that has much the more power as a disinfectant, (see Mechanics of inactivation, below). The proportion of each of these two forms is determined by the pH of the water. So pH is an important factor in making sure the free chlorine is an effective disinfectant.
The analysis of free chlorine using DPD solution methods cannot distinguish between the two components: it measures both HOCl and OCI-. So it is important to measure pH and apply a simple factor to calculate amount of active free chlorine that is available as a disinfectant.
The relationship between pH and the balance of the forms of free chlorine is shown below in the graph and table.

For ease of use, a simple factor can be applied to the free chlorine test result to calculate the active free chlorine present.
At a pH of:
- 7.0, 75% of the test result is active free chlorine
- 7.5, 50% of the test result is active free chlorine
- 8.0, 25% of the test result is active free chlorine
It is important to understand that the DPD test result has to be interpreted taking into account temperature as well as pH, to obtain the active free chlorine value. The table below is an illustration of this.
The level of active free chlorine (HOCl) from DPD free chlorine test and pH at a temperature of 25oC
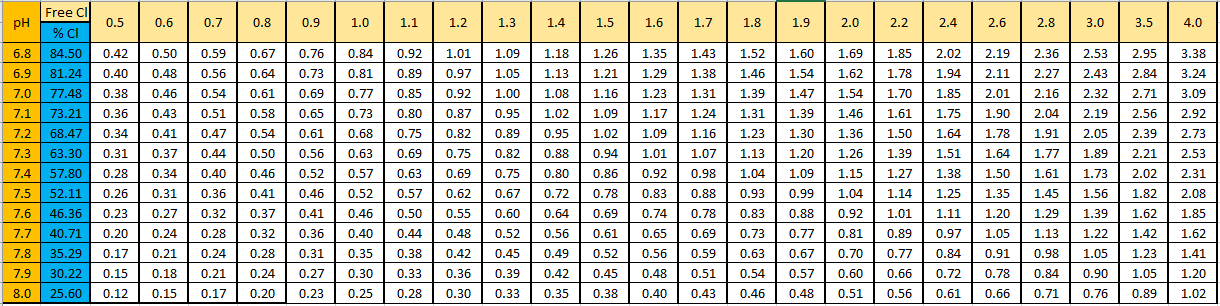 The DPD free chlorine test result is in yellow (mg/l)
The DPD free chlorine test result is in yellow (mg/l)
The pH test result is in orange
The results in white are the active free chlorine, ie HOCl (mg/l)
The mechanics of inactivation
It is generally not possible to explain precisely how a particular chlorine species inactivates a given microorganism but inactivation is believed to be by means of one or more of the following mechanisms: inactivation of key enzymes; disruption of nucleic acids, rendering them non-functional; and oxidative damage to cell walls or other vital cell components.
It is probable that there is a common sequence of events. This can be envisaged as interaction of the chlorine species with the cell surface followed by penetration into the cell and action at the target site(s).
Pathogenic microorganisms are effectively inactivated if they are unable to reproduce; it is not necessary to completely halt all of their metabolic activity (ie to actually ‘kill’ them) to prevent them from multiplying and causing disease.
For each of the inactivation mechanisms listed above the effectiveness of each disinfecting agent is a function of both its rate of diffusion through the cell wall and its reactivity with cells walls, enzymes and nucleic acids. From a practical standpoint, the specific inactivation mechanisms involved in disinfection are less important than the factors that influence the rate and extent of inactivation. These are known to include:
- the type and concentration of organisms being inactivated
- the disinfectant species present
- the concentration of disinfecting species
- contact time
- temperature
- pH
- interfering agents (particles and substances that exert a chlorine demand).
CT value
The disinfection requirements for potable water supplies and swimming pool water are typically based on CT values, calculated by multiplying the disinfectant concentration (C) by the contact time (T). Minimum values have been established for free and combined chlorine (as well as other disinfectants) for specific target organisms, as a function of the temperature and pH of the water.
The WHO Guidelines for drinking-water quality recommend 30min exposure to 0.5mg/l chlorine at a maximum pH of 8, which gives a CT value of 15mg.min/l to achieve effective disinfection. This seems a long time compared to swimming pools, but the continuous treatment in a recirculating pool allows for extended contact time. On the other hand, chlorine efficacy increases as pH value reduces: going from pH 8 down to 7.2 increases the CT value roughly threefold (and of course vice versa). And the higher temperature of pool water compared to drinking water increases the disinfection reaction of chlorine with bacteria roughly fourfold. So pool water disinfection should proceed rapidly enough in a well-run pool.
Hypochlorous acid HOCl
HOCl is overall the most effective disinfectant of the chlorine species present in dilute solution at the pH values associated with pool water treatment. Because HOCl is uncharged and has a relatively low molecular weight it is better able than other chlorine species to penetrate cell walls and membranes and it reacts more rapidly than other chlorine species, in both oxidative and substitution reactions with organic matter, including critical components of cells.
Since the dissociation of HOCl to form OCI-is strongly dependent on pH, and since OCl-is a much weaker disinfectant than HOCl, the germicidal efficiency of free chlorine depends very strongly on the pH. The table above shows the percentage of undissociated HOCl as a function of pH values and temperature. Lowering the pH increases the percentage of HOCI, generally enhancing disinfection.
Lowering the temperature suppresses HOCl dissociation, which would suppress disinfection if this were the only consideration. However, an increase in temperature will also increase the rate of diffusion of chlorine into cells, increase the rate of reaction with vital cell components, and increase metabolic activity (accelerating the toxic effects of chlorine on cells). On balance an increase in temperature actually increases the rate of inactivation rather than decreases it.
- Subject: Understanding and determining the importance of the active free chlorine content of the free chlorine test
- Date: December 2020
- Download Print Version: Technical Note 60 PDF
